Having established the real reason for the Greenhouse Effect (yes, there is one), we now have to address why the Greenhouse Effect is less marked than we'd predict using basic physics alone.
We'd predict a temperature at sea-level of about 300K and a temperature of about 200K at the top of the atmosphere, the precise numbers depending on what altitude you choose as starting point (where actual temperature is equal to effective temperature = 250 - 255K) and whether you assume dry lapse rate of 8K/km or 10K/km.
1. As it happens, the Greenhouse Effect is less than this - average temperature at sea-level 288K and average temperature at the top of the stratosphere 213K - the 'moist lapse rate' is 6.5K/km, not 8K/km or 10K/km.
So water vapour is reducing the Greenhouse Effect by about 12K at sea-level, and so it can't be a 'greenhouse gas' (to the extent there even is such a thing).
2. As a matter of fact, humid places not only have smaller day/night temperature ranges than drier places at the same altitude and latitude, they are also on average slightly cooler overall.
3. The main reasons for this are as follows:
a) When water evaporates from the surface, extra kinetic energy (heat) is converted to latent heat of evaporation/condensation. When water vapour condenses back to water droplets higher up, it converts latent heat of evaporation/condensation back to actual kinetic energy (heat). That's a one-way transfer of energy from surface to troposphere.
b) Clouds have a cooling effect in the day time (reflecting solar radiation so it never reaches the hard surface) and a warming effect at night (when clouds reflect some light and some infra red radiation back to the hard surface). That's a two-way thing, but the balance is a cooling effect (see point 2). To complicate matters, the cooling effect is stronger in summer than it is in winter (when it possibly has a slight warming effect).
4. The Consensus argues that water vapour (i.e. H2O in its gaseous 'dry' state, as distinct from water droplets in clouds or fog) absorbs infra red radiation which would otherwise leave the surface and go out to space. Some of this infra red is reflected back to the hard surface and warms the hard surface up a bit more. This is quite possibly true, but it is nigh impossible to split the overall 12K cooling effect into the stronger cooling effects (latent heat and day time clouds) and the weaker warming effects (night time clouds and reflected radiation) and any attempt to do so would be subject to so many estimates and intelligent guesses as to be meaningless.
5. The Consensus also argues that more water vapour will lead to a self-reinforcing cycle - higher hard surface temperature leads to more water vapour; more water vapour reflects more radiation to hard surface; this warms hard surface even more; etc etc. Logic tells us that this can't happen, or else it would have done long ago.
There is no need to speculate about what would happen if sea-level temperatures rise by one or two degrees - the tropics are tens of degrees warmer than the Poles, and most of the tropics are oceans with plenty of water available, and there hasn't been a self-reinforcing cycle there; the overall picture is that water vapour and actual water are a self-regulating, moderating influence.
During the Little Ice Age, temperatures were barely affected in the tropics but went down a lot at higher and lower latitudes. Since then, temperatures have bounced back more at very high and very low latitudes (i.e. North and South Pole, where there is very little water vapour) and haven't risen much in the tropics (very humid). Which again points to water vapour being a moderating influence.
Sunday, 31 May 2020
Why water can't be a 'greenhouse gas'
Posted by
Mark Wadsworth
at
18:24
7
comments
![]()
Saturday, 30 May 2020
Another little mystery solved!
Now that I have finally stumbled across the basic physics (which the Consensus keeps well hidden) that tells you 99% of what you need to know about why there has to be a lapse rate (and hence why the sea level temperature is warmer than you'd expect from solar radiation alone) and how to calculate it (between 8K/km and 10K/km, depending on which method you use - water vapour and water droplets moderate that to the observed 6.5K/km lapse rate), lots of other things just fall into place.
The infamous IPCC 'world energy budget' diagram (scroll down here) shows that the troposphere is emitting twice as much IR radiation back down to the hard surface as it is radiating to space. So with a straight face, the Consensus segues into the claims that 'greenhouse gases' are trapping heat and/or actually reflecting it, in other words the IR bounces back and forth between hard surface and troposphere, the 'greenhouse gases' act like a layer of insulation etc.
This all seems very implausible to me. In any sane world, a hot object emits IR evenly in all directions. The Consensus are also confusing cause and effect - the lower layers aren't hotter because they are absorbing and hence emitting more radiation; they are hotter anyway because of the inevitable lapse rate, so of course they emit more radiation!
The effect is actually measurable though, so it's a question of finding the actual explanation. There is no point picking holes in a flawed explanation (I wasted far too much time and mental effort on that), just start again with the basic physics (and get cause and effect the right way round!).
The actual explanation is - as ever - quite simple.
We've already established that the actual surface of Earth is not the hard surface, it is the troposphere (and the top few inches of land or ocean that are part of the troposphere for calculation purposes). The average overall temperature of the troposphere is - unsurprisingly - the same as the effective temperature' (the temperature you can calculate on the basis of the amount of solar radiation coming in adjusted for albedo, on the assumption that there is no atmosphere), which is 250-255K. The temperatures lower down are higher and the temperatures higher up are lower than the effective temperature, because of the lapse rate.
So the troposphere at sea level is 288K. This is - according to the Consensus, and I am taking their word for it - emitting twice as much radiation (half of which hits the ground) as the layer of the troposphere that is high enough to be emitting radiation directly to space (half of which actually goes to space).
You can easily work out the temperature of the layer higher up that is emitting radiation to space, and then you can work out its likely altitude.
Just divide sea level temperature (= 288K) by the fourth root of 'double' (assuming the "twice as much" figure to be reliable) (= 1.19) = 242K.
How high is that layer?
Deduct 242K from 288K (= 46K) and divide that by the expected/calculated lapse rate (between 8K/km and 10K/km, call it 9K/km for sake of argument) = 5.1 km.
The Consensus figure is "about 5 km", so we are not far off!
Posted by
Mark Wadsworth
at
15:38
10
comments
![]()
Thursday, 28 May 2020
Fun with numbers - where does the sunshine go?
To try and put the numbers into perspective, I did some workings.
Step 1. Multiply up the amount of incoming solar radiation per second (in Watts/m2, i.e. Joules/second/m2) to find out the total number of Joules each m2 gets in a 12-hour day (= 20.6 million of 'em).
Step 2. Look up mass/kg and specific heat capacity (Joules/kg/1 K) for air, wet soil, and water.
Step 3. Adjust/tweak the main variables until you get 'sensible answers' in the last column. The main variables are:
a) how those 20.6 million are split between air/soil and air/water. (answer 80/20 and 20/80 respectively, partly due to albedo and partly to do with how good soil and water are at moving heat downwards or back up into the air again)
b) the height of the column of air which noticeably warms during the day (answer 800 metres*)
c) how far down from the surface the soil warms up (answer 4.5 inches)
d) how far down from the surface the oceans warm up (answer 39 inches).
The 'sensible answers' are that the soil surface/the air above it warms by 16K during the day; the ocean surface/the air above it warms by 4K during the day - which is why in the day time you tend to get onshore breezes and at night you get offshore breezes. The Earth is two-thirds covered in oceans and that averages out to 8K.
* Clearly, there's not a clear cut-off of 800 metres altitude. So for example, maybe the lowest layer above the land warms by 16K, 800 metres up it warms by 8K etc, and at 1.6 km (about 1 mile up), the air barely changes temperature from day to night. Same applies to soil and water, going downwards. If you are building a sand castle, you don't have to dig very far down before the sand is noticeably colder than at the surface. Go for a swim in the afternoon, the top few inches are pleasantly warm; stand chest deep further out and your feet get cold.
At night, the reverse happens, and the lapse rate flattens again. In extreme cases, the land cools so far and so fast that it drags down the temperature above it so far and so fast that you get a temperature inversion, i.e. warmer air over colder air, that's like a negative lapse rate.

Which is all good fun, but what is the relevance?
Firstly, that you don't need to worry about quite how or why energy/heat is absorbed, transferred or distributed (conduction, convection/down drafts, mixing or wind/currents, radiation, latent heat of evaporation/condensation). The sun sends us a certain amount of total energy and it warms stuff up, and we can reconcile/estimate how much stuff is warmed up by how many degrees K. Sometimes the obvious answers are the correct ones and need little further investigation.
Secondly, what it reminds us that is the daily variation, based on incoming solar radiation, is relatively small compared to the absolute temperature. At its coldest (just before dawn), the surface is (say) 284K and at its warmest (mid/late afternoon) it's 292K.
Which, as ever, makes me question the Consensus obsession with this chart. That particular one is gloriously mislabelled as "Earth's annual and global average energy budget". It's not! That's the global average energy budget per second! They don't even understand their own propaganda.
What the Consensus is trying to do is explain that you can and should work out how many people are in a shop by looking at how many go in or come out every second (or in their terms, the average difference between the number people going in and coming out, which must be zero anyway, hence meaningless). Sure, it gives you a guide, but you'd also have to know roughly how long each person remains in there. If ten customers enter and exit a corner shop every hour, there will only be one or two customers in there at any one time. If ten customers enter and exit a large car show room every hour, there might be about ten customers in the show room at any one time. (Ignoring the lock down rules).
The "customers entering and exiting" are like the sunshine that arrives every day, which is sufficient to warm the soil/ocean surface and the air above it by 8K on an average day; it cools down again by 8K on an average night. The infamous chart gives you no clue whatsoever as to what the baseline average temperature is ("the number of customers actually in the shop").
So why not just count the actual number of people in the shop (the baseline average minimum temperature)?
The answer to this is not particularly difficult: stuff warms up and then it cools down again. Basic physics. The smaller the surface area relative to the volume/mass, the slower it is to warm up or cool down. Warmth from the sun can't get very deep into the soil or the ocean, so for each 1 m2 of surface, there's a column of troposphere with a volume of +/- 11,000 m3, which can only lose heat to space (counting the stratosphere and higher layers as 'space') via the 1 m2 at the top.
Most of the energy (kinetic energy, potential energy or latent heat of evaporation/condensation) in the air and top bit of land and oceans (which is effectively part of the atmosphere for these purposes) is left over from the previous day; and most of what was left over from the previous day was left over from the day before that ad infinitum. Mathematically, this energy has a half life of about 24 days.
-----------------------------------------------------------------
These ramblings have now reached full circle. My guess is that the Earth's atmosphere is set up to radiate a certain % of its energy every 24 hours. The equilibrium temperature is therefore where the extra sunlight that comes in during the 12-hour day is equal to the amount lost during the subsequent 12-hour night. If Earth's surface is radiating 2.78% (relative) every 24-hours, and is receives 8K's worth (absolute) when the sun is shining on it, then the equilibrium is 8K ÷ 0.0278 = 288K. Something like that.
Posted by
Mark Wadsworth
at
21:24
14
comments
![]()
Wednesday, 27 May 2020
Water, water everywhere
From the Arizona Daily Independent:
Water vapor is a powerful greenhouse gas, but its net effect in the atmosphere is to lower temperatures party because of convective heat transfer. Proponents of anthropogenic global warming (AGW) and most IPCC climate models assume the opposite (and that’s why climate model predictions diverge from reality).
AGW hypothesis: Carbon dioxide, a weak greenhouse gas, begins warming the planet. This warming evaporates water and so puts water vapor into the atmosphere which amplifies the warming effect. This is called a positive feedback.
At first look, this proposition seems logical and reasonable. But other properties of water vapor reduce temperatures and the net effect is a strong negative feedback. A positive feedback tends to destabilize a system, whereas, a negative feedback tends to keep a system in check. Just think for a minute, if water vapor had a net positive feedback effect, this planet would have had run-away global warming long ago. That alone should falsify the positive feedback hypothesis. But let’s look at some observational evidence for a negative feedback.
The graphic below compares four pairs of cities, each at about the same latitude so that each pair receives about the same amount of sunlight, and the cities are inland, away from possible tempering by sea breezes. The data is from the National Weather Service (the temperatures have been corrected for elevation differences). The difference between the pairs is that one city is in an arid climate, the other is in a humid climate. We see that the more humid city in each pair has a lower average annual temperature. The addition of water vapor to the atmosphere has a cooling effect in spite of water vapor being a greenhouse gas much more powerful than carbon dioxide.
Posted by
Mark Wadsworth
at
14:28
0
comments
![]()
Labels: Water
Monday, 25 May 2020
Climate science - as easy as A, B, C.
We all accept that the actual average sea-level temperature of the Earth (288K) is about 33K higher than its 'effective temperature' i.e. what it would be if Earth had no atmosphere (255K). That's basic physics - average incoming solar radiation in W/m2 minus amount reflected as light (known figures) raises temperature of the 'surface' (as defined - see below) to whatever it needs to be to radiate the same amount of W/m2 back out to space.
The Consensus is that the entire 33K discrepancy is due to the presence of minor trace gases in the atmosphere, such as the +/- 2% water vapour or 0.04% CO2 ('greenhouse gases'). The surface converts short wave visible radiation from the Sun to longer wave infra red radiation, and this is trapped/absorbed or reflected by the 'greenhouse gases', which in turn warms up the surface even more, so it emits even more infra red in a vicious circle. Here is a typical article explaining this, which includes the energy budget diagram which doesn't add up as a bonus. Parts of that explanation are correct, parts are guesswork and assumptions, it is riddled with contradictions, leaves a lot of loose ends and unanswered questions, and overlooks some important basic physics.
Actually, if you sift out the basic physics from the guesswork and just apply the basic physics, it is quite easy to work out that the hard surface should be 45K - 50K warmer than the effective temperature. The formulas and calculations are the same whether or not there are any 'greenhouse gases', which suggest that they do not increase temperatures. As a matter of fact, there is only a 33K discrepancy, because water vapour and water in the troposphere have a moderating influence and reduce surface warming and temperature fluctuations (which accords with everyday experience and like-for-like comparisons of temperatures in humid and dry areas).
They could and should teach this as part of GCSE level physics, it wouldn't take more than two or three lessons.
A. Barry
You can easily work out that there are about 10,000 kg of air (101,325 Pascal ÷ 9.807 m/s/s) for every m2 surface. We know that 1 m2 of air at sea-level has a mass of 1.293 kg. So if atmosphere were same pressure and density all the way up, with a hard edge, it would be 7.7 km high.
You can guess intuitively that the atmosphere gets thinner as you go up and gradually tapers off into space and that there is no hard edge. So let's assume actual average density is half that at sea-level, a reasonable guess is that most of the mass of the atmosphere is up to an altitude of 15 km or so, and atmospheric pressure falls by about 7% for each km you go up, at least for the first five or ten km (which is not far off actual measurements).
If you want to calculate this properly, you use the Barometric Formula (or 'Barry', as I affectionately call it) which is based on actual ideal gas laws and gives you reasonably accurate predictions for pressures at different altitude, at least for the troposphere (which is all we really care about, i.e. is the bottom 11 km, others say bottom 13 km, it's thicker at Equator and thinner at the Poles). The formula is very clever. I can just about understand how they work it out, but I would struggle to reverse engineer it or explain how to derive it.
B. The lapse rate
In an intuitive way, you can also guess that the temperature at the top of the atmosphere is close to the temperature of the nearly empty vacuum of space, which is either close to 0K or has no measurable temperature at all, depending on your point of view. So temperature falls the higher you go, as anybody who has been up a mountain knows.
Remember that 'energy cannot be created or destroyed, it merely changes from one form to another'. Air at sea level as thermal energy (aka kinetic energy) and no potential energy (it can't fall any further down). Air higher up has the same amount of total energy - less kinetic energy and some potential energy. It is reasonable to expect the total amount of energy to be the same at different altitudes.
Once you accept this, you can work out the lapse rate. I'll show you how, just for fun and because it is important:
Potential energy in Joules = mass x gravity x height.
So J = m x g x h
Joules required to increase temperature of 1 kg of a substance by 1K = specific heat capacity ('cp' ) of that substance.
So T = J ÷ (m x cp)
We can simplify/merge those two equations as follows: T = (m x g x h)/(m x cp); cancel 'm' top and bottom; T = g x h/cp; divide both sides by h; T/h = g/cp.
(Thanks to Tallbloke for this short-cut - Wiki gives an explanation which is almost impenetrable to the layman, although it ends with exactly the same formula)
1 kg of air which is 1,000 metres higher up has got 9,807 more Joules of potential energy than 1 kg 1,000 metres lower down, so the air lower down must have 9,807 more Joules of kinetic energy (and vice versa).
How much warmer is the air 1,000 metres lower down?
Specific heat capacity of air = 1,006 J used/needed to increase 1 kg of material by 1K
T/1,000 = 9.807/1,006
T/km = 9.75K
Hence the predicted lapse rate = 9.75 K/km altitude.
[I think it makes more sense to a) use the specific heat capacity for constant volume rather than constant pressure, and b) to calculate J/m3 rather than J/kg. That means first using Barry to find pressure, at different altitudes and then finding the temperatures which balances Joules of kinetic energy and Joules of potential energy (basing calculations on density at different altitudes, not on pressure, so you need to know pressure and temperature to work out density, and the temperature is the thing you are looking for!), which is why the lapse rate I worked out was 8K/km. I'm not sure if my logic on a) and b) is 100% sound, but my method gives an answer which is closer to the real world observed typical rate of 6.5 K/km, so I'm happy with my method for now.]
C. What is the surface of the Earth?
The Consensus give a nod to Barry and the main reason for the lapse rate. They don't deny they exist, they give the formulas but then downplay them as irrelevances and draw no conclusions from them. It's like the road sign saying turn left and the sat nav saying turn left, but turning right anyway. It's all about radiation from the surface being reflected back down by those dastardly 'greenhouse gases'! Any other explanation is heresy!
The Consensus' most heinous and borderline criminal obfuscation is in their definition of the 'surface'. They define 'the surface' as the hard surface at sea level, or the surface of the oceans (two-thirds of Earth's surface is ocean).
Here are a few reasons why that is wrong:
1. If you calculate the 'effective temperature', you should also be looking at 'effective surface', which is whatever the sun light hits first, Earth has a lot of clouds, so the 'effective surface' is NOT at sea-level.
2. If you approach Earth at speed from space, you feel it when you hit the atmosphere. That is the real surface.
3. If you scale down the earth to the size of a football, the troposphere is only 0.2 mm thick. You think it's thick when you look up at an airliner, but it's only 11 km away, you could drive that far in a few minutes.
4. There are about 10,000 kg of air (mass) for every m2 surface (see above)
5. The sun only warms the top few inches of the hard surface (or water), that's a few kg of mass per m2. So if the atmosphere is 0.2 mm thick, the hard surface or sea-level is barely a couple of molecules thick.
6. When we talk about the surface of the ocean, we mean the top of the water, not the hard surface at the bottom.
7. When we talk about the surface of the Sun or a Gas Giant, we mean the surface of the atmosphere, not the hard surface lower down (to the extent there even is one). Why do we change the rules when looking at rocky planets with thinner atmospheres?
8. As far as heat distribution goes, we might as well treat the top few inches of the hard surface as the bottom part of the troposphere. It's usually the same temperature, and must be the same pressure.
Therefore, the real surface is the whole troposphere, not the hard surface or the surface of the oceans.
Summary
The troposphere is the surface, and as a whole and on average, is the temperature you would expect from incoming solar radiation = +/- 255K. This is what you would expect, and this is what you get. I don't see why anybody taking this view should be on the defensive in a discussion. It is those trying to say otherwise who are struggling.
The troposphere itself, it is not a constant 255K. There has to be - and there is - a lapse rate. There are different ways of calculating/predicting it, and it can be observed/measured, so it's about 33K warmer than 255K at the bottom (the hard surface); it's about 255K half-way up (as defined); and it's about 33K cooler than 255K at the top of the troposphere.
Bonus
This A-B-C easy GCSE-level approach also explains a lot of things which the Consensus explanation can't and doesn't (and by and large, just glosses over to save embarrassment), for example:
1. Why the top of the troposphere (or the peak of a very high mountain) is colder than the 'effective' temperature, i.e. colder than it would be if Earth had no atmosphere.
2. Why the day/night temperature range on Earth (+/- 15K) is so much smaller than the day/night temperature range on the Moon (+/- 300K).
3. Why, despite the 'greenhouse effect', Earth's day-time temperatures at the surface are lower than what they would be without an atmosphere.
4. Why the 'greenhouse effect' is much stronger at night (i.e. actual temperature minus expected temperature of the night side of Earth if it had no atmosphere) than in the day time (when the Sun is blazing down on us and there is plenty of radiation sloshing about).
5. Why 'heat rises' is a truism only observed in enclosed spaces kept above the temperature of their surroundings (central heating in buildings; actual greenhouses/polytunnels) and why convection doesn't actually transfer heat upwards. For sure, there are thermals above hot surfaces (like square miles of dark tarmac at airports, which can make landing trickier than it need be on hot days), but for every molecule that goes up, one has to come down. One molecule converts kinetic energy to potential energy and the one coming down does exactly the opposite.
6. Why there is no need to get tied in knots over which methods energy (in its various forms - visible and infra red, kinetic energy, potential energy, latent heat of evaporation/condensation etc) is distributed in the troposphere (conduction, convection or radiation). All you need to know is that energy tries to distribute itself as evenly as possible (governed by the physical laws discussed here, or by winds/weather, if you want an everyday term for a complicated process).
7. Why the troposphere emits twice as much radiation towards the ground than it does out into space. This cannot be satisfactorily explained by 'greenhouse gases trapping and/or reflecting heat', it is because the troposphere at sea level is warmer and hence emits more radiation that the layer higher up, which is colder and so emits less radiation, see here.
8. Why water can't be a 'greenhouse gas', although this is based on observation rather than physics, which is really complicated with water vapour and water (see here).
9a. Why it is irrelevant that N2 or O2 are transparent to, and cannot absorb or emit infra red (even if this were true, which is questionable). They can certainly warm up, and they in turn keep the hard surface at the same temperature. So even if N2 and O2 aren't emitting infra red themselves, the hard surface converts that warmth back to radiation anyway. The total infra red leaving the hard surface is the same whether it is bouncing back and forth as infra red between hard surface and troposphere (the Consensus), or whether the hard surface has to convert kinetic energy from N2 and O2 back into infra red first (the actual explanation).
9b. Why, even if the troposphere were indeed completely transparent to and unaffected by infra red radiation, incapable of absorbing or emitting it, the temperature at the hard surface would be the same as it is now. The hard surface would quickly reach 255K (accepted by the Consensus) and it would warm up the troposphere by conduction and convection until the whole troposphere (the effective surface) were 255K on average (it being incapable of radiating heat to space, that's the Consensus). That 255K would be the average, there would still be a lapse rate and so the temperature of the hard surface would increase to 288K (and the top of the troposphere would be about +/- 222K). The hard surface would then be warm enough to emit the required amount of infra red straight through the troposphere and back into space (not being able to lose any more energy to the troposphere by conduction or convection).
10. Why there is a lapse rate on all planets with an atmosphere, even Gas Giants (Jupiter, Saturn), which have no hard surface (and if they do, radiation from the Sun never gets there) and which consist mainly of 'non-greenhouse gases' (mainly hydrogen and helium); why they are insanely hot at their centres; and why those Gas Giants are actually emitting more radiation to space than they get from the Sun.
11. Why the 'greenhouse effect' on Mars is barely measurable (max 5K), even though there is twenty-five times as much CO2/m2 surface area as there is on Earth.
12. Why you can predict Venus' hard-surface temperature fairly accurately using the same basic physics (here). All you need to know is distance from Sun and albedo, from which you work out 'effective temperature'; the height of 'effective surface'; the acceleration duty to gravity and specific heat capacity of the gases in the atmosphere, from which you work out the lapse rate up- and downwards. Whether or not the constituent gases are 'greenhouse gases' is entirely irrelevant. Knowing the mass of constituent gases in kg/m2 helps as well for cross checking.
Posted by
Mark Wadsworth
at
16:17
9
comments
![]()
Labels: greenhouse effect, Logic, Maths, Physics, Science, Weather
Sunday, 24 May 2020
Weekly deaths - all causes - E&W - 2020 - up to week 19
Data from the ONS.
My assumption is that at some stage later in the year, the red line will be undershooting the other two. But we shall see.
Posted by
Mark Wadsworth
at
13:44
10
comments
![]()
Labels: Covid-19, Death, statistics
Saturday, 23 May 2020
Hydroxychloroquine
This whole topic is fascinating.
Donald Trump just plucked it apparently out of nowhere. Outlets like The Guardian poured scorn on the idea (this article is a joy to read). Some doctors say it helps or might help; others say it's nonsense. A lot of people say they took it with no side effects (but we'll never hear from those took it and died). Many small scale trials have had mixed or inconclusive results.
Ever the contrarian, I'd be delighted if it does help. Even a stopped clock is right twice a day (or in Trump's case, a stopped calendar, he's right once a year). As a human being, I'd be delighted it they find anything that helps, I don't know or care what that might be.
However, it appears that some researchers are taking it seriously enough to do a very large scale trial. Possibly for the satisfaction of proving that Trump is an idiot, but hey. No experiment has ever 'failed'. You do them to find out whether anything happens and if so, what happens. So even if nothing happens or the opposite of what you expect happens, that experiment has not 'failed' on its own terms. It has advanced human knowledge, however slightly.
But... are these trials really necessary?
We've been running a large scale real-world trial for decades - the drug is regularly taken against malaria, and is taken by people suffering from lupus or rheumatoid arthritis. Why don't we just go back and find out what the COVID-19 infection and death rates are among the hundreds or thousands (millions?) of people who are taking it anyway for other reasons?
Posted by
Mark Wadsworth
at
16:24
15
comments
![]()
Labels: Covid-19, Donald trump
Friday, 22 May 2020
Missing figures - how to make the IPCC's figures add up
Their 'energy budget' was the topic of my two previous posts. If you summarise it, you get the following table:
That is the average for any 24-hour period in total. Problems arise if you try to dis-aggregate it into 'day' and 'night' (see previous post).
1. I think they didn't understand latent heat of evaporation (they show that the surface gives 80 W/m2 to the atmosphere) and the heat/potential energy transfers (they refer to this as 'thermals' and show that the surface gives 17 W/m2 to the troposphere).
Latent heat and potential energy can't be expressed in W/m2, they are expressed in Joules! They can't be measured with light sensitive equipment or with a thermometer! Some heat at the surface is converted to other forms of stored energy entirely.
Like trees - they capture a lot of light and some heat from the sun and convert it to stored energy i.e. chemical energy. This type of energy cannot be measured with light sensitive equipment or a thermometer! You can however convert this stored chemical energy back to a lot of heat and some light by burning it.
So while the surface is losing some its W/m2, those W/m2 are not being added to the troposphere, and they just drop out of the equation. When water condenses or there is a down draft (to match a rising thermal elsewhere), the energy is converted back to heat, which warms the surface, enabling it to radiate more Wm/2 again. There is no one-way transfer from surface to atmosphere, the W/m2 disappear from the surface and re-appear at the surface when and where the condensation and down drafts happen.
2. There are at least three more or less mutually exclusive theories as to what happens when infra red radiation hits a 'greenhouse gas' molecule and the diagram mixes and matches. It can't do all three things at once, and none of them actually increases the amount of energy:
A. Absorption
Molecule briefly absorbs the photon which increases the molecule's kinetic energy; before it has time to re-emit the photon, it collides with an N2 or O2 molecule, and increases the kinetic energy of those. We are told that all warm objects emit infra red except N2 and O2, so they are stuck with the higher kinetic energy*. This leads to warming. Fine, we know you can warm things up by shining infra red at them, so this in isolation makes sense.
(* Which leads me to a different train of thought, if N2 and O2 can only warm up - conduction and convection from the surface - but never radiate infra red, how would they ever cool down, even at the top of the atmosphere? If you think this through, the atmosphere would actually be a lot warmer than it is if it had no 'greenhouse gases'. Which is almost certainly nonsense, but shows up the theory to be nonsense as well.)
But, as with the conversion from actual heat to latent heat or potential energy, conversion from infra red radiation to heat takes those W/m2 out of the equation and parks the energy sideways in a different equation (same as latent heat, potential energy or trees). Temperature is measured in K, not in W/m2.
The diagram does not take this into account, so it actually shows no warming effect.
B. Scattering or re-radiation
The molecule absorbs and re-emits the infra red photon, so it scatters it or re-radiates it at random in all directions. Fine, this also makes sense in isolation.
If you start with a random photon somewhere in the troposphere moving in a random direction, you know that there are slightly more molecules per unit volume beneath it than above it. Therefore - you would reasonably assume - it is ever so slightly more likely that it will be bounced upwards by a molecule below it than being bounced downwards by a molecule above it.
Analogy - in the pub, people crowd round the bar. You are standing quietly drinking your pint. If you move away from anybody who jostles you, you will end up at the other side of the room. But I don't know how to adjust for this - the atmosphere is radiating a total of 199 W/m2 to space, so let's assume it is also radiating 199 W/m2 back to the surface.
C. Reflection
The theory appears to be that the molecule reflects the infra red photon directly back down again, the same way that is gets noticeably warmer (and slightly brighter, if you are in a back-lit urban area) on a clear night when a large thick cloud passes you at the right altitude.
This seems the least plausible theory.
But the diagram relies heavily on the theory of reflection. Out of the 332 W/m2 'back radiation', 199 W/m2 can be explained by theory B. above, meaning the average extra 133 W/m2 must be reflected - it is the only way to reconcile their figures.
It's worse when you dis-aggregate it into day/night: by day, the atmosphere is reflecting 13% straight back down, which seems just about plausible. But at night, the atmosphere is reflecting 45% straight back down, which is clearly nonsense.
Put the two together, and that's why they show an ugly grey band of 'greenhouse gases' at cloud level which appears to bounce 85% of the radiation from the surface straight back down again.
3. But never mind, I'm assuming that enough of their numbers are honestly and accurately measured or calculated for my version to be plausible - at least on their terms. I have used their figures as far as possible, but I had to change thermals from 17 W/m2 to 31 W/m2.
If the table looks a bit blurry, click it to see it clearly.
Posted by
Mark Wadsworth
at
16:47
8
comments
![]()
Thursday, 21 May 2020
Missing figures round - how do you make the IPCC's figures add up?
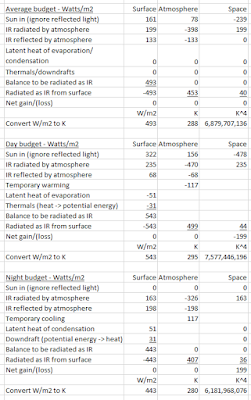
Here's our baseline energy budget, averaged over 24 hours. I have been battling with this for the past 48 hours (hence no post yesterday) and have pinned down its fatal flaw:

We see that the Earth's surface receives, on average, 493 W/m2, consisting of 161 incoming solar radiation (ignore the one-eighth that is reflected straight back) plus 333 back radiation. The average temperature of the surface is 288K. That's our starting point.
If we had no atmosphere, the Earth's surface would receive 298 W/m2 incoming solar radiation, i.e. the 341 minus the one-eighth that would be reflected straight back, and its temperature would be about 250K - 255K (opinions differ). That's another fixed point.
So as a check, to see if we are on the right track, let's see if we can what calculate Earth's average surface temperature would be without an atmosphere.
You convert W/m2 to temperature in K as follows. You need to have a starting position (493 W/m2 and 288K). If W/m2 change, then the change in temperature to the fourth power is proportional to the change in W/m2.
298/493 = 0.605
0.605 x 288K ^ 4 (6.88 billion) = 4.16 billion
4.16 billion ^ 0.25 = 254K.
Excellent! It is widely agreed that the average temperature of the Earth, if it had no atmosphere, would be around 254K, so it stacks up so far.
Now, let's do the same exercise for night-time and day-time (working backward from temperature to find W/m2).
Night-time
Typical average Earth night-time temperature is (say) 7C = 280K.
280K^4/288K^4 = 0.893. 0.893 x 493 W/m2= 441 W/m2.
Therefore, Earth's surface must be receiving 441 W/m2 at night.
At night, the earth is clearly getting no incoming solar radiation.
Let's assume the back radiation is the same (333 W/m2).
That gives us a missing figure of 108 W/m2.
Does anybody know where this missing 108 W/m2 comes from?
Day-time
To have an average day-time temperature of (say) 22C = 295K, total incoming radiation must be 541 W/m2 (work it out yourself).
It's seems fair to assume that during the day, the Earth's surface is getting twice the average incoming solar radiation (that is how it is calculated) = 161 x 2 = 322.
The Earth's surface must be getting at least as much back radiation by day as it does on average = 333 W/m2.
That's a total of 655 W/m2 incoming. This would give an average day-time temperature of 309K = 36C, which is clearly nonsense.
To get the 541 W/m2, the amount of back radiation must be 114 W/m2 less than the average, which is also clearly nonsense.
------------------------------------------------------
UPDATE - I think I have cracked it, see next post.
------------------------------------------------------
IMHO, the whole thing is a load of nonsense, it just crumbles under closer inspection, so using it to try and reconcile know and sensible figures is like trying to prove that the square root of the colour blue equals a banana.
The correct way to explain/reconcile Earth's average surface temperature with incoming solar radiation - which works by day or by night, with or without an atmosphere - is intuitive, relatively easy to calculate (once you know how), and completely different to their model.
So my next challenge is to re-state all their figures to reconcile W/m2 and temperature for the overall average position; the position if we had no atmosphere; night-time and day-time. I think I know what they missed, possibly deliberately (i.e. what they missed is the latent heat of evaporation/condensation and the temperature/potential energy trade offs when air rises or falls). But maybe I'm wrong :-)
Posted by
Mark Wadsworth
at
17:11
8
comments
![]()
Tuesday, 19 May 2020
Sunny side up!
From researchgate.net, a diagram showing how the total amount of radiation received at the Earth's surface is more than incoming solar radiation:

Fair enough, this implies that day time temperatures are higher because of The Greenhouse Effect. Seems plausible. If you don't think about it for more than a few seconds.
Problem is, it ignores facts.
Our Moon is the same distance from The Sun as we are. The max daytime temperature is 127C and the night-time low is -173C (from here). That's a day-night range of 300C. Why is this? It is because our Moon has no atmosphere.
There's no official adjusted average for day-night range on Earth (wildly different for dry deserts and humid cities) but let's go daytime average 20C and average night time 5C? A range of only 15C.
So the actual warming effect of the atmosphere is far stronger at night, when the Earth's surface is 178C* warmer than it would be without an atmosphere (i.e. same as Moon's night-time temperature).
The flip side of this is that the Earth's day-time temperature is 107C* cooler than it would be without an atmosphere (i.e. same as Moon's daytime temperature)- exactly when these diagrams tell us that the surface should be warmer than it would be without an atmosphere/without The Greenhouse Effect.
Back to the drawing board, lads! Maybe redo those diagrams to explain why The Greenhouse Effect cools the surface of the Earth during the day? (You can't, it's as logically impossible as what the above chart is trying - and clearly failing - to explain).
* Sure, on average the Earth's surface is about 35C warmer than our Moon's surface. That's because we have an atmosphere, full stop, regardless of its constituent gases.
Posted by
Mark Wadsworth
at
21:57
2
comments
![]()
Labels: Science
Monday, 18 May 2020
Suffolk May Morning Birdsong
Posted by
Lola
at
14:24
3
comments
![]()
Labels: Birds
Sunday, 17 May 2020
Tesco tills are in the key of A#
The three notes I can pick out are A#, C and D. If anybody has more info or background, I'd love to know it!
Posted by
Mark Wadsworth
at
20:37
6
comments
![]()
"Lecture 6: Stability [in the atmosphere]"
After much Googling, I have finally found somebody who looks at the bigger picture. From The University of California, Irvine (pdf), slide #5:
What Happens to the Temperature?
• Air molecules in the parcel (or the balloon) have to use their kinetic energy to expand the parcel/balloon
• Therefore, the molecules lose [kinetic] energy and slow down their motions
=> The temperature of the air parcel (or balloon) decreases with elevation...
All the dime store explanations stop right there and ignore the obvious follow-on questions:
- What happens to the heat aka kinetic energy of the legendary 'parcel of air' once it has risen?
- What happens to the temperature of the air that was higher up that is displaced and falls?
These lecture notes cover it very succinctly:
...=> The lost energy is used to increase the potential energy of air molecules.
• Similarly when the air parcel descends, the potential energy of air molecules is converted back to kinetic energy.
=> Air temperature rises.
That kinetic energy isn't "lost", it is just converted to a different type of energy - potential energy. That is the missing figure you need to get the whole atmosphere to balance, despite being the easiest to calculate (mass x height x gravity).
For every parcel that rises, an equal and opposite amount of air must fall elsewhere, which compresses it and causes it to warm up (for two subtly different reasons; the compression itself does not cause the warming, it is the 'falling' that causes both). Which is most of the reason that air is warmer at sea level. The dime store explanation that the sun hits the ground, warms it up and this warms up the air above it etc is only a small part of the bigger picture (it ignores... cloudy days; night time; the fact that two-thirds of the Earth's surface is ocean and stays nearly the same temperature 24/7; and the fact that the Sun shines with the same intensity on the top of mountains).
----------------------------
If this seems far fetched, let me give a real life example:
How can you use water to store electricity?
Clearly you can't, not directly. But you can do the next best thing and use spare night time electricity to pump water up into a reservoir (convert electrical energy to potential energy), then at times of peak demand, you use the water in the reservoir to generate generate hydro-electricity (convert potential energy back into electrical energy).
The air in the atmosphere does exactly the same trade-off, only the trade-off is between heat aka kinetic energy and potential energy.
Posted by
Mark Wadsworth
at
12:55
12
comments
![]()
Saturday, 16 May 2020
Burning Down The House

On one level, that's a pile of wood.
But you can also see it as "stored chemical energy". If and when I burn it, I will be releasing energy from the sun that was trapped and converted to wood a century ago. By reversing the process that happened a century ago, I will be warming the atmosphere slightly.
Light energy -> chemical energy -> light energy and heat.
So the reverse must also hold. A century ago, the tree was cooling the atmosphere by converting light energy (which otherwise would have been converted to heat) into chemical energy. That's why it's cooler in woods and forests, and why allowing them to grow will cool the atmosphere, however slightly. (The fact that they also trap CO2 is a very minor issue).
Posted by
Mark Wadsworth
at
20:39
17
comments
![]()
Labels: Fire
Friday, 15 May 2020
Council Tax non-logic
Home-Owner-Ist logic says that "council tax pays for local services", which is why there is a single person's discount and many councils offer discounts for second homes and empty homes (unused, derelict or being refurbished).
Fair enough.
On the other hand, a few years ago, Cornwall decided that leaving homes empty is a waste of housing, so imposes a council tax surcharge of 50% on them and also scrapped the second home discount because locals are being driven away. A few years after that, the Welsh Assembly decided enough was enough and allows local councils to impose a surcharge of up to 100% on empty homes and second homes.
So we have two entirely opposite policies for the same factual situation.
There is no need for a Single Person's Discount. By and large, they live in (or should be living in) smaller homes with a lower Council Tax bill. Families will be in a larger home and pay more Council Tax anyway.
What is the fundamental difference between:
a) a single person owns a home with some spare bedrooms in the same house (and gets a discount)
and
b) a single person lives in a flat and owns a second home somewhere else with some spare bedrooms (for which some councils give a discount and others impose a surcharge)?
Or, taking this to extremes (Bayard's real life example), a single person lives in a house and gets a 25% Single Person's Discount on the whole thing. If he converts his house into two flats, he only gets the 25% discount on the flat he lives in and has to pay (up to) double on the other one.
Far better and consistent to have Land Value Tax at a flat rate on everything. If one household owns a house worth £300,000, they would pay the much same amount of tax as another household which lives in a £200,000 house and owns a £100,000 holiday home. And I see absolutely no reason why one household should pay more (or less) than the other one.
Posted by
Mark Wadsworth
at
14:10
11
comments
![]()
Labels: Council Tax
Thursday, 14 May 2020
Cars and Garages
Thank you to everyone who took part in the Fun Online Poll, good turnout.
Which of the following apply to you (multiple answers allowed)?
Total - 86 voters
I have no garage - 40 votes
I use my garage to store junk - 28 votes
I use my garage as a workshop - 15 votes
I keep my everyday car in my garage - 8 votes
I keep my second car in my garage - 7 votes
I use my garage for some other purpose (please specify)* - 7 votes
My garage has been converted to living space - 2 votes
With only 8 out of 46 people using their garage to keep their everyday car in, my suspicions that most people have repurposed their garage is confirmed. I'm also glad to see that my usage is the most popular one.
Only one person actually specified: Frank said "I store my bicycles in my garage and I have a set of rollers to ride my bike when I can't go out. I also use it to store my garden power tools: lawnmower, strimmer etc."
Posted by
Bayard
at
13:51
5
comments
![]()
Call for council tax relief on empty landlord properties
https://www.property118.com/call-for-council-tax-relief-on-empty-landlord-properties
Predictable that the NRLA would ask for this. All sorts of things wrong of course. Interestingly, many councils just don't bother trying to get a landlord to pay a few days council tax between tenancies. It makes sense as the cost of collection is not worth it in many cases but I wonder how much this costs councils? FOI request if I have time...
Posted by
mombers
at
12:55
7
comments
![]()
Monday, 11 May 2020
Yup, as I already said.
For example here.
From the BBC:
People working in social care in England and Wales have been twice as likely to die with coronavirus as the general working-age population, Office for National Statistics figures show.
But [NHS] workers have been no more likely to die than other workers.
People in care homes and care workers have been treated as the poor relatives in all this*. Yes, a couple of hundred NHS workers have died, which all goes into the "NHS as heroes" myth, but if you look at the actual numbers, strip out the retired NHS workers who were press-ganged back into service and died*, in deaths/100,000 workers, nurses and doctors have the same (surprisingly low) chance of dying of COVID-19 as the general working age population.
So it appears that very few NHS workers were infected "in the line of duty", or at least no more than anybody else who gets infected while commuting or at work.
* Two actual bloody outrages, if you ask me.
Posted by
Mark Wadsworth
at
17:25
30
comments
![]()
Sunday, 10 May 2020
Weekly deaths, England & Wales, all causes, week 1 to 17
Duly updated. It does look as if the headline 30,000 coronavirus-related deaths are all incremental additional deaths, not (as I was expecting) overlapping with deaths that would have occurred anyway.

Posted by
Mark Wadsworth
at
14:07
16
comments
![]()
Labels: Covid-19, Death, statistics
Random conversation with a care home worker
I got chatting to the lady shuffling along 2m behind me in the supermarket queue.
The conversation turned to the coronavirus, as you might expect, and she told me she worked in a care home. There are sixty or seventy regular staff, many of whom also visit other care homes. The last time she was tested was three or four weeks ago.
She agreed that this was an absolute disgrace and a disaster waiting to happen (or already happening).
Posted by
Mark Wadsworth
at
13:56
5
comments
![]()
Labels: Covid-19
Saturday, 9 May 2020
How to work out the 'adiabatic lapse rate' using logic and maths (instead of relying on observations).
Some of my posts drag on a bit before the conclusion, so this time I will do bullet points first (with links to skip down to the fuller explanation).
1) The traditional explanation, that the sun warms the Earth's surface; the surface warms the air above it is largely nonsense, or is only a small part of the picture, if you bother thinking about it for a few minutes. Fuller explanation.
2) The actual explanation is that energy tries to spread out and equalise in all directions. The air at the surface has no potential energy but lots of heat. Further up, the air has more potential energy but less heat, all the way up to the top of the troposphere. The two forms of energy (potential plus heat per unit volume) is a constant all the way up. Fuller explanation.
3) Some constants and formulae we have to accept as a given; we plug these into the workings; and work backwards to get the lapse rate that balances Joules/m3 all the way up. Constants, formulae and workings.
4) Conclusion: to get the Joules/m3 to balance all the way up (I calculated up to 6km, half-way up), the local temperatures of the troposphere has to fall by 8C (or 8K) for every 1 km you go up. The extra potential energy = the energy no longer needed to warm the air in each m3.
That's a bit more than the observed lapse rate of 6.5C, and a bit less than the 9.8C which Wiki says is the theoretical dry lapse rate. So I'm pretty happy with it for now.
5) How to set up hyperlinks within one web page, from ComputerHope.com.
-----------------------------------------
1) The traditional explanation is (largely) nonsense.
You're seven years old and your science teacher tells you that the Sun warms the earth surface; this warms the air above it; that warms the air above that etc. So the bottom layer is warmest and it gets colder the higher you go. So kids, she trills, that's why it's so cold at the top of Mt Everest.
Sounds plausible until you relate it to real life...
- there is a lapse rate at night, when the surface is not being warmed and it's actually cooling down faster than the air. Which is why you should have more layers underneath you than above you when sleeping outside.
- there is a lapse rate, even when it's cloudy and the surface is not being warmed; the sun is hitting the clouds first. It's still colder above the clouds than below.
- the Sun (when it's shining) hits the top of Mt Everest with the same intensity as it hits land at sea level; but it doesn't warm it up nearly as much.
- the surface cools down from average 293K by day to average 273K at night (let's say for sake of example) and warms to 293 again the next day. So 3.5% of the heat is lost and regained each day, it has a 'half life' of about three weeks. Most of the heat is left over from previous days and that has had plenty of time to mix evenly (if it wanted to do so).
- it's like saying "Bath water is deeper at the tap end, because that's where the water goes in." Wrong, the surface quickly levels off. Bath water is indeed deeper at the tap end, but it's because that's where the plug hole is, and the plug hole end is lower.
- Yes, on a sunny day, you can see hot air rising from e.g. dark tarmac = 'thermals'. Vultures and hang glider pilots use these to get around. But this is a dynamic thing, it disturbs the equilibrium, which is constantly trying to re-establish itself, thermals are not the equilibrium or an explanation for it.
2) The actual explanation.
- I hope we all remember "Energy cannot be created or destroyed, it just transforms from one from to another". The sun sends us energy as light; plants use it to grow and they store chemical energy. You burn wood (stored chemical energy) in a power station and convert it to heat; that turns water to steam; steam gets converted to kinetic energy and water (again); the kinetic energy gets converted via magnetic to electrical energy. Which goes down the wires, through a filament in a bulb and becomes light energy and heat; the same as what we got from sun a long time ago.
- energy will choose whatever form (radiation/light, magnetism, electrical, heat, sound etc) enables it to spread out as evenly and as quickly as possible for a given medium.
- heat will flow from warm to cold (speed depends on how good the medium insulates); photons will go from light to dark (which is why it takes them so long to leave the sun; it's equally bright everywhere and they're not sure which way to go); once the photons have escaped the sun, they whizz off at the speed of light; sound waves travel as fast as they can from loud to quiet (760 mph through the air at sea level; faster through solids).
- gases, i.e. the atmosphere, are very compliant. Energy can travel through it by conduction; by radiation; or by convection i.e. converting heat to potential energy (air will carry sound and shock waves, and even conduct electricity if the potential difference is high enough = lightning). The precise method and speed are immaterial in an equilibrium situation. They all boil down to the same thing and the speed is to all intents and purposes instantaneous. If heat from the atmosphere hits the edge of the atmosphere, it doesn't care, it turns into light/radiation and keeps going, the only way for energy to travel through a vacuum.
- on a more mundane level, a hot air balloon is a way of converting heat energy to potential energy (altitude). If it were 100% efficient, you would gain exactly the same amount of Joules in potential energy as you use up in Joules of heat energy. The troposphere is as close to 100% efficient as you can get.
- so it seems reasonable to assume that the total energy per unit volume (like a m3) will be the same at all altitudes in the troposphere in an (idealised) equilibrium situation, let's call this 'energy equivalence' for the sake of calling it something.
3) The constants, formulae and workings.
The main steps:
1. Set up your spreadsheets with all the scientific constants and formulae.
2. Find out surface/sea level temperature and plug that into Barry to find the altitude at which pressure = 0.5 atm, which is 5.9 km.
3. Take 0.5^(1/5.9) to find the % by which pressure falls for each km altitude.
4. Work out total Joules/m3 at sea level.
5. By trial and error, find the temperatures/lapse rate which give you the closest amount of Joules/m3 all the way up. You get the best fit with 8K per 1,000 metres.
So the result of Barry feeds into this calculation and vice versa. Doesn't matter, it is not a circular calculation if there is an equilibrium and the results match up to observations.
Bottom row shows I'm pretty close up to 5 km and only 3% out at 6 km altitude. The measured temperature half-way up is apparently 250K, not 240K. But overall, I call it a good result for a couple of hours with a calculator in the back garden. Click to enlarge.

Posted by
Mark Wadsworth
at
21:11
9
comments
![]()
Thursday, 7 May 2020
Is there life on Mars?
I doubt it, but what I do know is that it has an atmosphere:
Despite the high concentration of CO2 in the Martian atmosphere [95%], the greenhouse effect is relatively weak on Mars (about 5°C) because of the low concentration of water vapour and low atmospheric pressure. While water vapour in Earth's atmosphere has the largest contribution to greenhouse effect on modern Earth, it is present in only very low concentration in the Martian atmosphere.
So the CO2 makes little difference then? It's the water vapour all of a sudden? (Venus also has hardly any water vapour and is 95% CO2).
What makes the big difference is the low atmospheric pressure, but they only mention that in passing. On the surface of Mars this is only 0.61% of sea level atmospheric pressure on Earth (and on the surface of Venus it is 92 times as much).
The effective temperature of Mars, i.e. its surface temperature if it had no atmosphere, is 210K. It's actual average surface temperature is 215K. To get the Barometric Formula to balance and give you an average temperature of 210K for the whole atmosphere*, you have to put in a surface temperatures between 215 and 218K.
This is not far off the official figure and gives you a lapse rate of a bit less than -1K/km altitude. NASA say it is almost exactly -1K/km. And they've been there and measured it. Interestingly, the altitude/temperature chart in the Wiki article (first link) also shows a lapse rate of about -1K/km, even though the article itself says actual -2.5K/km and predicted -4.3K/km,
So the formula comes through for us yet again; all that matters is that a mole of CO2 has an atomic mass of 44, about one-and-a-half times the mass of a mole of the Earth's atmosphere (mix of N2 and O2 = 29). You don't need to know anything else about the gases' properties.
As an approximation, density and pressure are proportional, so Mars has about fourteen times as many CO2 molecules per unit volume as Earth does. Earth = 420 ppm; Mars = 1,000,000 x 0.61% x 95% = 5,800.
So this next bit is a real cop-out:
Moreover, under low atmospheric pressure, greenhouse gases cannot absorb infrared radiation effectively because the pressure-broadening effect is weak.
CO2 molecules are a lot closer to each other on Mars than on Earth!
* Earth's effective temperature is given as 255K, which is very close to the average temperature of the atmosphere, depending on how it is calculated. So let's assume that the the average temperature of a planet's atmosphere is equal to its effective temperature. The charts tell us that the surface temperature is higher than the average and the temperature at the top of the stratosphere is lower than the average.
Posted by
Mark Wadsworth
at
17:52
15
comments
![]()
Labels: global warming, Science
Wednesday, 6 May 2020
Somebody please make it stop!!
Exhibit A, from the BBC:
There are natural fluctuations in the climate but scientists say temperatures are now rising faster than at many other times.
This is linked to the greenhouse effect, which describes how the Earth's atmosphere traps some of the Sun's energy. Solar energy radiating back to space from the Earth's surface is absorbed by greenhouse gases and re-emitted in all directions.
This heats both the lower atmosphere and the surface of the planet. Without this effect, the Earth would be about 30C colder and hostile to life.
1. As ever, they say "the Earth would be about 30C colder" instead of "the Earth's surface would be about 30C colder".
2. The Earth's atmosphere is, on average, about 30C cooler than the Earth's surface. They always gloss over that bit. The lowest part (where it touches the surface) is 30C warmer than average and the highest part is 30C cooler than the average.
3. The Barometric Formula explains that there has to be a temperature gradient going up ('adiabatic lapse rate' in Newspeak).
4. After we'd done out Physics O Level, the teacher said, let's stretch our mental legs a bit, and did some clever workings to show that if you know atmospheric pressure and density of air at sea level, you can extrapolate upwards to see where the top of the troposphere is, which contains 99% of the atmosphere (it gets a bit mad further up). I can't remember how he did it, but it all stacked up. To cut a long story short, pressure falls at a fairly constant rate, so it's not difficult to estimate pressure at a given height if you know pressure at sea level and that it's effectively zero 11 km up. And temperature also falls at a fairly constant rate. That's two of the variables you need for the formula without breaking a sweat. Expected temperature then just pops out of the formula, and is surprisingly close to observed results.
5. If the facts fitted the BBC's "really simple guide", then not only would the surface be 30C warmer, the top of Mt Everest would also be warmer - but it's not, it's a lot colder. They hedge their bets and fudge it with this claim: "This heats both the lower atmosphere and the surface of the planet." Are they trying to say that the top of Mt Everest would be warmer if there were no CO2?
6. To use a crude analogy, a perfectly efficient fridge doesn't change the average temperature of your kitchen. It causes the inside to be colder and warms up the rest of the kitchen to balance. The atmosphere does the same sort of thing. It can't change its own average temperature (how?), that's dictated by the Sun, but it cools the top half and warms the bottom half in equal and opposite measure.
--------------------------------------------------------
Exhibit B, from Wikipedia:
The temperature of the troposphere generally decreases as altitude increases. The rate at which the temperature decreases, -dT/dz, is called the environmental lapse rate (ELR). The ELR is nothing more than the difference in temperature between the surface and the tropopause divided by the height. The ELR assumes that the air is perfectly still, i.e. that there is no mixing of the layers of air from vertical convection, nor winds that would create turbulence and hence mixing of the layers of air.
The table below gives an ELR of 6.5C/km. You can measure this directly or work it out by plugging height and pressure into the formula. Rather infuriatingly, they give a variant of the formula in the section above, but don't draw any conclusions from it.
The reason for this temperature difference is that the ground absorbs most of the sun's energy, which then heats the lower levels of the atmosphere with which it is in contact. Meanwhile, the radiation of heat at the top of the atmosphere results in the cooling of that part of the atmosphere.
Sure, direct sunlight heats up the ground and that must warm the air directly above it. So the equilibrium is disturbed and tries to re-establish itself. But that is moving the goal posts. How much of the earth's surface is getting direct sunlight at any one point in time? It can't be more than half (day-night) and in the day time, maybe there's direct sunlight half the time (clouds). So overall, one-quarter? And two-thirds of the Earth's surface is water, which warms (and cools) much more slowly that the land.
Nonetheless, the temperature gradient is much the same by day or by night, when it could be argued that the surface cools the air above it. It is the same above the oceans (fairly constant surface temperature, day or night); it is the same above land (warms faster by day; cools faster by night); it is the same whether it's sunny or cloudy.
-----------------------------------------------------
We know all this stuff, or can easily find out. Why do they keep trotting out explanations that contradict each other and are completely at odds with known facts and basic physics?
(As ever, for sure, we also know that CO2 blocks/reflects infra red at a few frequencies, but that is a very marginal effect, no more than one degree here or there. There is twenty times as much C02 in the Martian atmosphere, but it is still bloody cold because it is further from the Sun, obviously, but also because the Martian atmosphere is only one-hundredth as thick as Earth's. The fact that it is 95% CO2 is largely irrelevant).
Posted by
Mark Wadsworth
at
13:24
17
comments
![]()
Labels: Science
Tuesday, 5 May 2020
More climate change fun with the BBC
From the BBC:
More than three billion people will be living in places with "near un-liveable" temperatures by 2070, according to a new study.
Unless greenhouse gas emissions fall, large numbers of people will experience average temperatures hotter than 29C. This is considered outside the climate "niche" in which humans have thrived for the past 6,000 years...
Researchers used data from United Nations population projections and a 3C warming scenario based on the expected global rise in temperature. A UN report found that even with countries keeping to the Paris climate agreement, the world was on course for a 3C rise.
According to the study, human populations are concentrated into narrow climate bands with most people residing in places where the average temperature is about 11-15C. A smaller number of people live in areas with an average temperature of 20-25C.
Ho hum.
From National Geographic:
The tropics are regions of the Earth that lie roughly in the middle of the globe. The tropics between [sic] the latitude lines of the Tropic of Cancer and the Tropic of Capricorn. The tropics include the Equator and parts of North America, South America, Africa, Asia, and Australia.
The tropics account for 36 percent of the Earth's landmass and are home to about a third of the world's people.
So the hot areas between the tropics have much the same population density as north and south thereof. They are not concentrated into "narrow climate bands", they are at all latitudes (except the Arctic circles and deserts. And half of the world's population (3.5 billion people) live within this circle, which is almost entirely between the tropics (the emptiest bit of that circle is the half of PR China, which is north of the Tropic of Cancer).
The tropics are warm all year, averaging 25 to 28 degrees Celsius (77 to 82 degrees Fahrenheit). This is because the tropics get more exposure to the sun. Because of all that sun, the tropics don't experience the kind of seasons the rest of the Earth does.
So one source says "A smaller number of people live in areas with an average temperature of 20-25C" and the other says that one-third of the world's population lives in areas with average temperatures of 25 - 28C. Which source looks more reliable?
If you add an arbitrary increase, like 3C, you can predict that average temperatures will be about 29C. Easy. I'm sure there are already plenty of inhabited areas with average temperatures of 29C, if it's so terrible, why do people live there?
And so on.
Posted by
Mark Wadsworth
at
16:11
13
comments
![]()
Labels: global warming
They own land! Give them money!
Apropos my comment on the last post "They own land! Give them money!", from The Guardian:
London NHS Nightingale hospital will shut next week
No 10 says decision is due to limited demand, with no coronavirus admissions expected in coming days
The showpiece Nightingale hospital in London will shut next week after treating a small number of patients but will be kept “in hibernation” in case a second wave of Covid-19 infections emerges.
No further patients will be admitted to the facility, which was created amid much acclaim in just 10 days, and the 12 patients being treated there at the moment are being transferred to other London hospitals...
Originally planned to have 4,000 beds, the Nightingale has treated just 54 patients since it was opened by Prince Charles on 3 April and received its first patient on 7 April. It has not admitted a new patient for a week as London hospitals have had spare capacity in their own intensive care units.
The four other Nightingales that were opened to stop hospitals being overwhelmed – in Manchester, Birmingham, Bristol and Harrogate – will also be wound down, though the London hospital will shut first. All were conceived in March, when ministers and health service bosses were concerned that NHS hospitals risked being overwhelmed by significant numbers of people needing to be ventilated to keep them alive, as Italy was confronting at the time.
But while the Manchester hospital has taken some patients, its sister facilities in Birmingham, Bristol and Harrogate have not admitted anyone.
Monday, 4 May 2020
Give that man some medals!
From The Daily Mail:
A son has managed to nurse his coronavirus-stricken father back to health at home despite the 81-year-old being released from hospital to die.
Raj Nathwani revealed his Suri [sic] went into Watford General Hospital on March 26 with suspected coronavirus after struggling on his daily walk. Doctors said they were 95 per cent sure Suri, who has chronic obstructive pulmonary disease, had the deadly bug. But they wanted to discharge him.
Raj used a Google spreadsheet to monitor Suri's vital signs, kept his house clean and isolated him from the rest of the family in Watford. The 55-year-old relied on a continuous positive airway pressure, also called 'black boxes', which are used to tackle sleep apnoea.
Hats off for his devotion, initiative, courage etc, but the key is the last bit (in bold).
I read an article somewhere or other, probably in a covid-conspiracy Whatsapp group, by a doctor who said that using ventilators was overkill and actually made matters worse. The gist of it was that it's not the virus that kills you, it's your immune response. Injuring the airways and lungs by intubating and then overloading them with oxygen exacerbates things. The survival rate of people who are put on ventilators is apparently quite appalling (although maybe it is not a fair comparison - they only put people on ventilators as a last resort).
The article concluded that the common-or-garden face masks (which people use to treat sleep apnoea) which increase the air pressure slightly was were much more appropriate (they are also a lot cheaper, easier to use, safer etc).
In this instance, it looks like the author of that article is on to something, and Mr Nathwani did exactly the right thing.
Posted by
Mark Wadsworth
at
15:10
8
comments
![]()
Labels: Covid-19
Sunday, 3 May 2020
They own land! Give them money!
It had to happen, I suppose...
From the FT:
In a letter to Rishi Sunak, the chancellor, a coalition of the UK’s biggest retail chains and property owners warned that “many viable companies” will file for administration if the government does not intervene further, causing job losses and a “devastating impact” on high streets.
“We have come together, as voices of both commercial tenants and landlords, to propose that the government introduces a scheme of rental support for the space that has in effect been furloughed, just as staff have been,” said the letter, from the chiefs of the British Retail Consortium and the British Property Federation...
The two industries want the government to support a “furloughed space grant scheme” where the state would cover the fixed costs of businesses that have experienced dramatic falls in turnover. Similar schemes have been set up in Denmark and other European countries.
The groups have proposed a sliding scale of partial payments to cover property costs, which would still leave some of the burden on the tenant and landlord.
Posted by
Mark Wadsworth
at
12:53
8
comments
![]()
Fun Online Polls: cars and garages
Suggested by Bayard.
Vote below; or here; or use the widget in the sidebar.
Posted by
Mark Wadsworth
at
11:31
1 comments
![]()
Friday, 1 May 2020
Killer Arguments for Raising the Rent, Not
Down in the West Country, a railway company is trying to bring in more income by raising the rent:
Their reasons include these gems:
"This rent is considerably below what the PLC (the railway company) feel is its value, and as a reference (1) the rent for Williton/Sherrings Yard is £17000 pa; (2) WSRA at Bishops Lydeard (BL) pay a rent of £4,500.00 pa for their office space; (3) the WSSRT pay a rent for the BL Museum of £100.00 pa paid in 4 instalments and (4) for the Blue Anchor Museum it is £741.60 pa paid in 4 instalments, however this has been waived until Oct 21 to assist the refurbishment. (5) Quantock House at BL was set at £22,000 pa, but then withdrawn by the landlord. (6) 5542Ltd pay £10 per foot of siding space pa."
As we know, what sets the rentable value of land is two things. By far the greater one is location. The other is what stands on the land. The site in question is in a small village with access only over a working railway off a tight bend in a major road. The tenants originally rented a bare site, with no buildings, rails or services on it and have paid for all the infrastructure themselves. To compare this site with a yard in an industrial estate (1) in a large village/small town with good level access is a classic diagonal comparison.
As is it to compare the rentable value of an empty site with that of a building, (items 2-5). Even in example (6), 5542 Ltd. didn't install the rails themselves. Moreover, as we also know, what the landlord feels is the value of the rent is neither here nor there, the value of the rent is what someone else would be prepared to pay for it.
The railway company also think that the needs of the landlord have a bearing on the rentable value. A large part of the statement from it is along the lines of "the company needs money, therefore the rent must go up".
"The PLC met the Trust and made clear it needed to increase its income wherever possible. It was suggested that the Trust should review its finances, that a rent increase was needed and that as this would take some time to manage, the PLC would accept a staged increase over time, but that an increase was needed."
The final piece of wishful thinking is the suggestion that the rent was originally set at below market levels out of benevolence, however there is no attempt at justifying this statement apart from the slightly ludicrous :
"At that time the PLC agreed the original lease it was trading profitably and could afford to be more generous than now; the level set is also an indication of the historic weak financial management which has been part the PLC’s current financial situation."
Just because it could, doesn't mean that it did and just because the Plc is in financial trouble now because of "weak financial management" (even if that really is the cause) doesn't mean that was the case back in 1991.
3/10 - must try harder
Posted by
Bayard
at
19:15
15
comments
![]()
Labels: Rents
Killer Arguments Against Citizen's Income, Not (28)
I'm sure I've covered this before, but it has raised its ugly head again (@CentreThinkTank on Twitter, who are otherwise pretty sound on all sorts of issues).
For some reason, they (like a lot of centre-right people) think Negative Income Tax is better than Citizen's Income because it can be 'targeted' at lower earners and so it is cheaper than paying it to everybody, including people 'who don't need it'.
As Sam Bowman at the Adam Smith Institute explains, The Negative Income Tax and Basic Income are pretty much the same thing.
His article is logically and mathematically correct, but I find it helpful to look at a real-life example...
A real life example
Ireland used to have* a hybrid system as follows, which I think is the best combination of the two:
1. Unemployment benefit was €300 a month.
2. There was, officially, no income-tax free personal allowance.
3. People in work received a 'tax credit' of €300 per month against their PAYE liabilities.
4. PAYE was calculated as follows:
step 1: multiply gross pay by 30% on all earnings (up to the higher rate tax threshold) = PAYE due.
step 2: reduce each person's PAYE by €300 each month (but not below zero)
5. If an unemployed person took on some some part-time work, he continued to receive the unemployment benefit but didn't get the tax credit as well. He had PAYE deducted at the flat 30% on all his earnings.
Worked examples:
The unemployed person gets net income €300 a month.
An unemployed person takes on some part-time work, €500 gross a month. Net of 30% tax he earns €350 and keeps his €300 unemployment benefit. Net income €650.
A low earner on exactly €1,000 a month looks at his payslip and sees PAYE deducted zero (the 30% x €1,000 and the tax credit exactly cancel out), net pay €1,000.
A medium earner on €2,500 a month looks at his payslip and sees PAYE deducted €450 (30% x €2,500 = €750, less €300 tax credit).
Conclusion
The final outcome (in terms of net income) is exactly the same whether you call the €300 'unemployment benefit' or 'tax credit' or 'negative income tax', or 'citizen's income'.
The 30% is the same whether you call it 'means-testing' or 'claw-back of unemployment benefit' or 'income tax'.
The majority earning more than €1,000 a month couldn't care less if you call it a €300 'tax credit' or a €1,000 'tax-free personal allowance'.
The part-timer on €500 a month should be indifferent whether he gets net pay €350 and €300 unemployment benefit; or whether he gets €500 gross pay and €150 negative income tax (being 30% x the excess of the notional tax-free personal allowance over his gross pay).
The difference here is psychological. We want the unemployed to take on low-paid or part-time work. If we say "You can keep your €300 unemployment benefit and keep 70% of your gross earnings", they won't miss the 30% tax deducted because they never had it. That is more of an incentive than saying "You can keep all the €500 you earn, but we will make you fill in some forms and will reduce your unemployment benefit by half".
It is impossible to say whether the Irish system was more like Citizen's Income or more like Negative Income Tax. Because in terms of the final outcome, they are identical and indistinguishable!!
It's just a question of administrative simplicity and minimising fraud and error. With NIT, you need a separate system for calculating NIT and clawing it back; with CI, the claimant just gets a BR tax code, so pays tax on all his earnings - the clawing back is done via the normal PAYE system. So the CI system makes life easier for the minority at the margins of employment, and those are the people we should be concerned with. For the long term-unemployed and the majority earning more than €1,000 a month, NIT and CI are the same.
With the Irish system, there would have been some people in work who continued to receive the unemployment benefit and get the tax credit. Boo. And there would have been some people who didn't get unemployment benefit and earned less than €1,000 a month, who didn't get the full value of the tax credit. Also boo.
Well, sure, but there will always be fraud and error in a welfare system and there will always be tax evasion if you try to tax output and earnings. And payroll departments and the tax office will always make a few mistakes.
----------------------------------------------------
* I've simplified this a bit and am doing this from memory. It's now more complicated, but you can recognise the basic model.
Posted by
Mark Wadsworth
at
15:47
0
comments
![]()
Labels: KCN, negative income tax